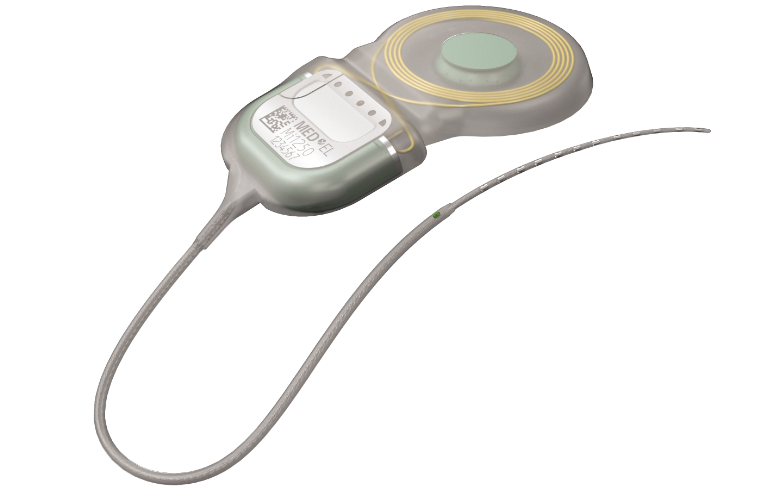
SYNCHRONY 2 for EAS
A great Electric Acoustic Stimulation system depends on a great implant. Find out how SYNCHRONY 2 is personally tailored to your hearing, to give you the sound quality you deserve.


1
Advanced Technology
SYNCHRONY 2 uses advanced sound technology, helping you to hear the world as it was meant to sound.
2
Soft Electrodes
SYNCHRONY 2 uses soft and flexible electrodes to protect your residual hearing and provide great sound quality.
3
Simple Surgery
SYNCHRONY 2 has been designed for simple surgery, helping you to get back on your feet afterwards.
4
easyMRI
Experience hassle-free MRIs with SYNCHRONY 2—no surgery*, no discomfort**, and no hearing downtime.
5
Reliable Design
SYNCHRONY 2’s safe and reliable design means you can benefit from years of great hearing.

An Important Decision
It’s important to choose the right audio processor, but it’s even more important to choose the right implant. Why? Because it’s the implant that most influences how well you hear.
SYNCHRONY 2 uses advanced coding strategies, designed to give you the closest to natural hearing of any CI system. This helps you hear the high and low tones, and the fine details of both everyday conversations and your favorite songs.

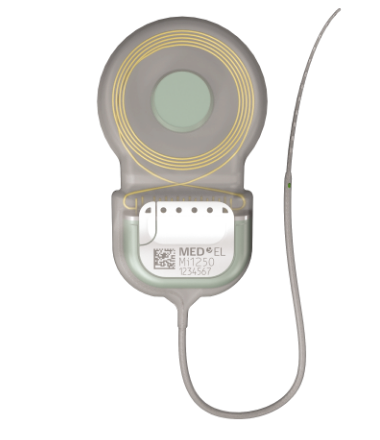
The Ideal Electrode
EAS is designed for people who can still hear some low-frequency sounds. Your EAS system uses cochlear implant technology to complement this natural “residual” hearing, helping you to hear better overall.
During surgery, your surgeon will try to preserve as much of your residual hearing as possible as this gives you better hearing with EAS. To help them with this, SYNCHRONY 2 only uses very soft and flexible electrodes. These have been specially developed to protect your cochlea during surgery, so you can keep as much of your residual hearing as possible, and hear your best with EAS.

An Easy Surgery
No one likes having surgery. Thankfully, our SYNCHRONY implants*** are not only the smallest and lightest titanium implants available, but SYNCHRONY 2 has also been designed to make your surgery as simple as possible. After an easy surgery, you can get back on your feet, and start enjoying the next stage of your hearing journey.


Made For MRI
You never know what life will bring, and statistically every one of us will need an MRI at some point in our lives. With some implants, this can mean extra surgery to remove the magnet in your implant, plus time without your audio processor as you recover from the surgery.
But not with SYNCHRONY 2. It contains a revolutionary kind of magnet that allows you to have MRIs of up to 3.0 Tesla without magnet removal surgery.* In fact, the way you have an MRI is no different with or without a hearing implant.

Great Protection. Guaranteed.
Because of our long and positive experience with MRIs and cochlear implants, we offer a life-long MRI guarantee. We promise to replace your implant in the very unlikely event that it’s damaged during an MRI scan.
This life-long MRI guarantee for cochlear implants is the first and only to be offered by any company and covers all MED-EL cochlear implants from as far back as 1994. So you can feel confident about any future MRI scans.
The Reliable Choice
Your implant will be with you for many years to come, so it should be reliable enough to provide you with years of use.
Like all MED-EL implants, SYNCHRONY 2 has been crafted to the highest standards. Each part is designed and tested for maximum reliability, before being manufactured in our European headquarters. And every implant has to pass our first-class quality inspection before arriving at your clinic.
All of this means you can expect the highest levels of reliability from your new SYNCHRONY 2.


Be Flexible. Be Free.
Smaller and lighter than SONNET 2 EAS and equipped with integrated direct streaming, SONNET 3 EAS offers you freedom and flexibility. Experience closest to natural hearing with our latest behind-the-ear audio processor.
We’re Here for You on Your Hearing Journey
Would you like to find out more about how MED-EL’s hearing solutions could help you or someone you care for? Simply fill out the contact form and we will provide you with further information.
Technical Data

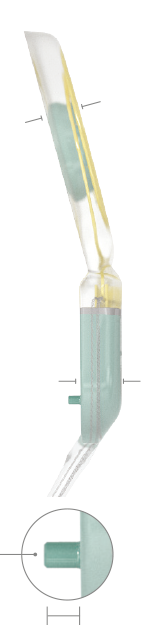
SYNCHRONY 2 Pin
Cochlear Implant (Mi1250)
Stimulation Features
- Sequential non-overlapping stimulation on 12 electrode channels
- Simultaneous (parallel) stimulation on 2 to 12 electrode channels
- 24 independent current sources
- Stimulation reference electrode on titanium housing
- Stimulation rates of up to 50,704 pulses per second
- Range of pulse phase duration: 2.1–425.0 μs/phase
- Time resolution (nominal values): 1.67 μs
- Current range (nominal value): 0–1200 μA per pulse phase
Pulse Shapes
- Biphasic, symmetric triphasic and triphasic precision pulses
Comprehensive Diagnostic Toolkit
- Status Telemetry
- Impedance and Field Telemetry (IFT)
- Electrophysiology measurements reference electrode on titanium housing
- Auditory Nerve Response Telemetry (ART™)
- Electrically Evoked Auditory Brainstem Response (EABR)
- Electrically Evoked Stapedius Reflex Threshold (ESRT)
- Electric Acoustic Evoked Potential (EAEP)
Housing Design
- Impact resistance up to 2.5 Joule
- Unique PIN variant with fixation pins for additional stability
- Hermetically sealed titanium housing
- Stimulator: 18.8 mm x 24 mm x 4.5 mm
- Coil: 29.0 mm diameter x 3.3 mm thick (typical)
- Weight: 7.7 g
Safety Features
- Independent safety capacitors for each electrode channel
- Unique Implant ID (IRIS)
- Biocompatibility standard according to ISO 10993-1
- Latex-free****
MRI Conditions*****
- MR Conditional at 0.2, 1.0, 1.5 and 3.0 Tesla
- No magnet removal required even at 3.0 Tesla
Removable S-Vector Magnet
- Removeable S-Vector magnet for minimized image distortion
- Rotatable magnet within hermetic titanium housing
- Self-aligning to external magnetic field
- Conical shape for secure placement
Electrode Arrays
FLEX Series
The softest and most flexible electrode arrays, designed for Structure Preservation and Complete Cochlear Coverage. Featuring 19 active and physical platinum electrode contacts and FLEX-tip technology for atraumatic insertion. All FLEX series electrodes feature a green orientation marker for improved visibility and positioning during insertion.
FLEX34
- 28.6 mm stimulation range
- Diameter at basal end: 1.3 mm
- Dimensions at apical end: 0.5 x 0.4 mm
FLEXSOFT
- 26.4 mm stimulation range
- Diameter at basal end: 1.3 mm
- Dimensions at apical end: 0.5 x 0.4 mm
FLEX28
- 23.1 mm stimulation range
- Diameter at basal end: 0.8 mm
- Dimensions at apical end: 0.5 x 0.4 mm
FLEX26
- 20.9 mm stimulation range
- Diameter at basal end: 0.8 mm
- Dimensions at apical end: 0.5 x 0.3 mm
FLEX24
- 20.9 mm stimulation range
- Diameter at basal end: 0.8 mm
- Dimensions at apical end: 0.5 x 0.3 mm
FLEX20
- 15.4 mm stimulation range
- Diameter at basal end: 0.8 mm
- Dimensions at apical end: 0.5 x 0.3 mm
FORM Series
Designed specifically for malformed cochleae and for instances where leakage of cerebrospinal fluid (CSF) is expected. Featuring 24 active and physical platinum electrode contacts and SEAL technology designed to aid closing of the cochlear opening.
FORM24
- 18.7 mm stimulation range
- Diameter at basal end: 0.8 mm
- Diameter at apical end: 0.5 mm
FORM19
- 14.3 mm stimulation range
- Diameter at basal end: 0.8 mm
- Diameter at apical end: 0.5 mm
CLASSIC Series
Features 24 active and physical platinum electrode contacts.
STANDARD
- 26.4 mm stimulation range
- Diameter at basal end: 1.3 mm
- Diameter at apical end: 0.5 mm
MEDIUM
- 20.9 mm stimulation range
- Diameter at basal end: 0.8 mm
- Diameter at apical end: 0.5 mm
COMPRESSED
- 12.1 mm stimulation range
- Diameter at basal end: 0.7 mm
- Diameter at apical end: 0.5 mm
* Unless required for diagnostic reasons.
** SYNCHRONY 2 has a self-aligning magnet, so there’s no painful magnetic pulling on the implant during an MRI scan.
*** MED-EL's SYNCHRONY implants include SYNCHRONY, SYNCHRONY PIN, SYNCHRONY
2, and SYNCHRONY 2 PIN.
**** Whereby “free” means “not made with latex” according to current FDA guidance.
***** It has been demonstrated that no known hazards exist in specified MRI environments under conditions as described in the SYNCHRONY 2 cochlear implant product labeling. The SYNCHRONY 2 cochlear implant is MR conditional. Recipients with
a SYNCHRONY 2 cochlear implant may be safely MRI scanned at 0.2, 1.0, 1.5, and 3.0 Tesla following the conditions detailed in the instructions for use.

