Reliable Hearing by Design: MED-EL Cochlear Implants
Hearing connects us to life’s cherished moments. That’s why we’re here to help people with hearing loss experience wonderful moments day after day.

360° Safety and Reliability
Since the very beginning, our life-changing devices have been developed with a complete 360-degree view of cochlear implant reliability and safety in mind. It’s not only about making our cochlear implants and audio processors as reliable as possible. Electrode design, safe stimulation, and MRI safety* are also essential for cochlear implant safety. Without considering these aspects, creating a safe and reliable hearing experience just isn’t possible.
Every MED-EL recipient puts their trust in us. We know you count on us to hear everything you love every day, and we’re committed to providing you with reliable cochlear implants that meet the highest quality and safety standards on the market.

Our complete 360° approach to cochlear implant safety and reliability focuses on five critical areas:
Implant reliability includes both manufacturer-reported data as well as data reported by clinics in peer-reviewed publications.
Electrodes shouldn’t harm the delicate structures in the cochlea while providing effective stimulation.
Stimulation must be safe and exclude harmful direct electrical currents.
Modern cochlear implants should provide you with the possibility to undergo MRI scans without the need for surgery,** and without hearing downtime.
Audio processor reliability includes both reports on repair rates as well as the audio processor not falling off your head.

Leading in Cochlear Implant Reliability
All of our latest-generation titanium implants have an overall cumulative survival rate of over 99%—reliability performance that no other cochlear implant manufacturer has achieved. [ft][ft][ft]
It is not by chance that our cochlear implants come with outstanding reliability. It’s the result of the focused oversight and expert craftsmanship that takes place in our state-of-the-art manufacturing facilities. Unlike other cochlear implant producers who outsource manufacturing overseas, every single MED-EL implant is produced at our global headquarters in Austria—and it has been that way for over 30 years. That way we can always maintain the highest standards of European quality and engineering.

The Value of a Reliable Implant
Choosing a cochlear implant can be a decision for life. With a MED-EL cochlear implant, you’ll have hearing you can always count on. Higher implant reliability can lead to a lower risk of additional surgery and higher satisfaction.
The Cumulative Survival Rate (CSR) is the measure of how reliable each implant model is over time. For example, a CSR of 99% within seven years means that the probability of your cochlear implant providing continued benefits after seven years is 99%.

MED-EL Cochlear Implant Reliability
MED-EL reliability reporting is compliant with the International Standard ISO 5841-2:2014[ft] and the principles set out in the European Consensus on Cochlear Implant Failures and Explantations.[ft]
With all of our latest-generation titanium implants since 2006 having an overall cumulative survival rate of over 99%, MED-EL leads in cochlear implant reliability.[ft][ft][ft]
MED-EL, Data on file as of July 1, 2024

Reliable Cochlear Implants You Can Trust
SYNCHRONY 2 has one of the best cochlear implant reliability performance records on the market, with an overall CSR of 99.90% within five years.[ft][ft][ft]
CSR within 5 years

A Deaf Ear Is Not a Dead Ear
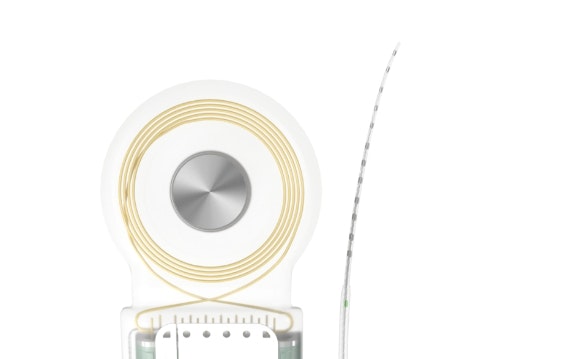
Your cochlea is an organ with extremely delicate structures. Preserving it’s natural function is important since you or your loved one may have some remaining natural hearing, known as residual hearing. Keeping these delicate structures intact is important because it may support your performance with your cochlear implant today, and it also may enable you to benefit from future technologies. [ft][ft][ft][ft][ft][ft][ft] Our competitors often use stiff electrode arrays, that can either be straight or pre-curved, which are far more likely to cause damage to delicate cochlear membranes, which can result in loss of residual hearing.[ft][ft][ft][ft][ft]
Unlike those arrays, MED-EL’s electrode arrays are flexible. This allows them to be inserted into the cochlea without harming those delicate structures. In a clinical trial of adult recipients of MED-EL’s Electric Acoustic Stimulation (EAS) system, which uses both a cochlear implant combined with a built-in hearing aid in the same ear and therefore relies on preserving residual hearing, 97% of the study participants were able to be fit with the acoustic component, and 97% also demonstrated benefit from the EAS device as measured on tests of speech recognition improvement, subjective improvement, or both.[ft]
Safe Stimulation
When it comes to long-term cochlear implant safety, safe stimulation is an absolute must. Our cochlear implants have safety capacitors that safeguard the cochlea from direct current that could damage neural tissue.[ft][ft]
Unlike some cochlear implant manufacturers, MED-EL has an independent safety capacitor for every electrode channel. That way, we can ensure safe, precise, and fast stimulation that’s ready to deliver more advanced sound coding for many years to come. [ft][ft]

Made for MRI
With most people needing an MRI in the next 10 years, we believe that every single hearing implant should be designed for outstanding MRI safety. That is why we created the first cochlear implant that offers outstanding safety during 1.5 and 3.0 Tesla MRI scans.
Other implants may require surgery to remove the magnet of the implant before an MRI, or risk pain, discomfort, or damage to the implant.* [ft] [ft]
With our SYNCHRONY 2 cochlear implant, there’s no surgery** and no hearing downtime. You’ll have peace of mind knowing you’re ready for anything life brings.


Great Protection. Guaranteed.
Because of our long and positive experience with MRIs and cochlear implants, we offer a life-long MRI guarantee. We promise to replace your implant in the very unlikely event that it’s damaged during an MRI scan.
This life-long MRI guarantee for cochlear implants is the first and only to be offered by any company and covers all MED-EL cochlear implants from as far back as 1994. So you can feel confident about any future MRI scans.
Reliable Hearing for Everyday Activities
It’s not just our implants that are designed to deliver outstanding reliability. Our audio processors are, too. You need a working external audio processor to be able to hear with an implant. With a reliable audio processor, you or your loved one will have reliable hearing for everyday activities.

Reliable Audio Processors
Our latest generation audio processors have a proven record of outstanding reliability. In fact, the average monthly repair rate[ft] of all our audio processors is low.[ft]
If an audio processor has an average monthly repair rate of 0.32%, it means that, on average, 32 audio processors have been returned for repair each month out of 10,000 total audio processors sold in the world by the end of the period covered.
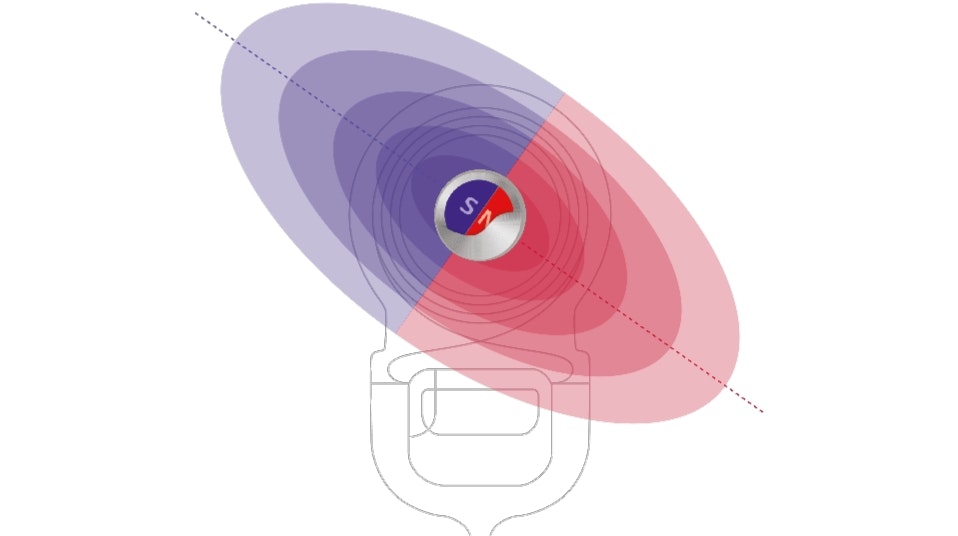
Optimized to Stay Securely in Place
Audio processor retention is important and often overlooked. MED-EL audio processors are optimized to stay securely in place thanks to our unique S-Vector magnet technology. Our new S-Vector magnet is 25% stronger so you can trust that your audio processor won’t fall off—especially if you enjoy sports like running or swimming or have a job where you often suddenly move your head.

Reliable Hearing You Can Trust
From the very beginning, MED-EL has been guided by our founders, the inventors of the modern micro-electronic multichannel cochlear implant. Our mission is to overcome hearing loss as a barrier to communication and quality of life.
To us, reliability is not a percentage on an earnings report for investors. Every recipient is a person, not a number. When you choose MED-EL, we know you are putting your trust in our hands, so we make sure we are always there for you.
* MED-EL cochlear implants since 1994 are MR conditional. Recipients with a MED-EL cochlear implant may be safely MRI scanned following the conditions detailed in the instructions for use.
** Unless required for diagnostic reasons.

